Understanding the Quantum Universe: Debunking 10 Common Misconceptions
Written on
10 Myths About The Quantum Universe
Even physicists sometimes fall for these.
For centuries, the principles of physics appeared entirely deterministic. If one had complete knowledge of every particle's position, velocity, and the forces acting upon them at a given moment, predicting their future behavior seemed feasible. From Newton to Maxwell, the laws governing the universe appeared devoid of any intrinsic uncertainty. Limits were solely due to our restricted knowledge, measurement capabilities, and computational resources.
However, this perspective shifted over a century ago. Discoveries related to radioactivity, the photoelectric effect, and light behavior in double-slit experiments revealed that, in many situations, we could only determine the probabilities of various outcomes due to the quantum characteristics of our universe. Alongside this novel and often perplexing view of reality, numerous myths and misunderstandings have emerged. Here are the truths behind ten of them.

1.) Quantum effects occur solely at small scales. When contemplating quantum phenomena, we often envision individual particles (or waves) and their strange characteristics. Yet, significant macroscopic effects are inherently quantum in nature. Superconducting materials, for instance, lose all electrical resistance when cooled below a certain threshold. Creating superconducting tracks for magnets to levitate and glide along has become a common student experiment, showcasing fundamental quantum effects.
Superfluids and quantum drums, which can simultaneously vibrate and remain still, represent macroscopic quantum phenomena that have garnered six Nobel Prizes over the past 25 years.
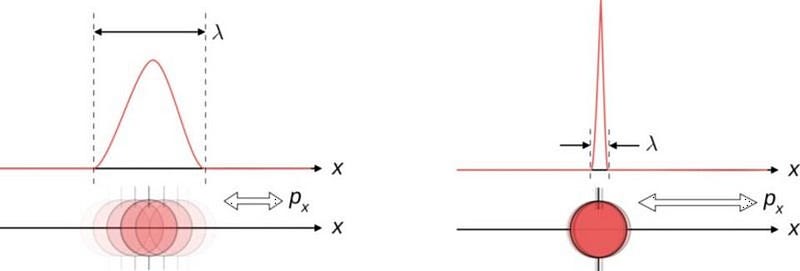
2.) Quantum implies “discrete.” The concept of partitioning matter (or energy) into distinct units—quanta—is crucial in physics, but it doesn't fully capture the essence of being "quantum." For instance, consider an atom comprising a nucleus with electrons. The question arises: where is the electron at any given moment? Even though an electron represents a quantum entity, its location remains uncertain until measured. When numerous atoms bond in a conductor, discrete energy levels emerge for electrons, yet their locations can vary widely within the material. Many quantum phenomena exhibit continuous qualities, suggesting that space and time might also be continuous at a fundamental level.
3.) Quantum entanglement enables faster-than-light communication. Consider the following experiment: - Create two entangled particles, - Separate them by a significant distance, - Measure specific quantum traits (like spin) of one particle, - Instantly gain insights about the state of the other particle.
However, no information traverses faster than light. Instead, measuring one particle constrains the probable outcomes of the other. If someone measures the second particle, they won't know whether the first has been measured or whether the entanglement has ceased. The only way to determine this is to compare both measurements, which can only be done at light speed or slower. In 1993, this concept was formally validated.
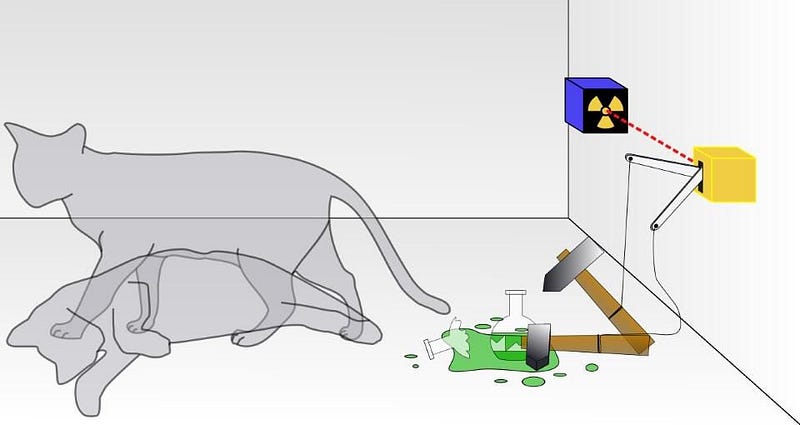
4.) Superposition is a core principle of quantum physics. Imagine a system capable of existing in multiple potential quantum states. You might find it in state “A” with 55% probability, “B” with 30%, and “C” with 15%. Yet, during measurement, only one state is observed: either “A,” “B,” or “C.” Superpositions serve as valuable intermediary steps for calculating potential outcomes and their probabilities, but we cannot measure them directly. Moreover, superpositions do not uniformly apply across all measurable variables; for example, superpositions can manifest for momentum but not positions.
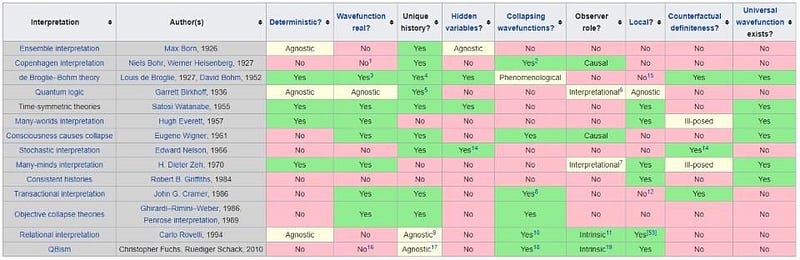
5.) It’s acceptable to have personal preferences regarding quantum interpretations. Physics revolves around what can be predicted, observed, and measured in our universe. Yet, in quantum physics, several interpretations exist that align equally with experimental results. Possible realities include: - Quantum wavefunctions collapsing upon measurement, - An infinite ensemble of quantum waves with measurements selecting one, - A superposition of potentials converging in a "quantum handshake," - A multitude of possible worlds corresponding to different outcomes.
Choosing one interpretation over another offers little insight beyond reflecting our biases. It's more beneficial to focus on observable phenomena rather than preferring an interpretation lacking experimental advantage.
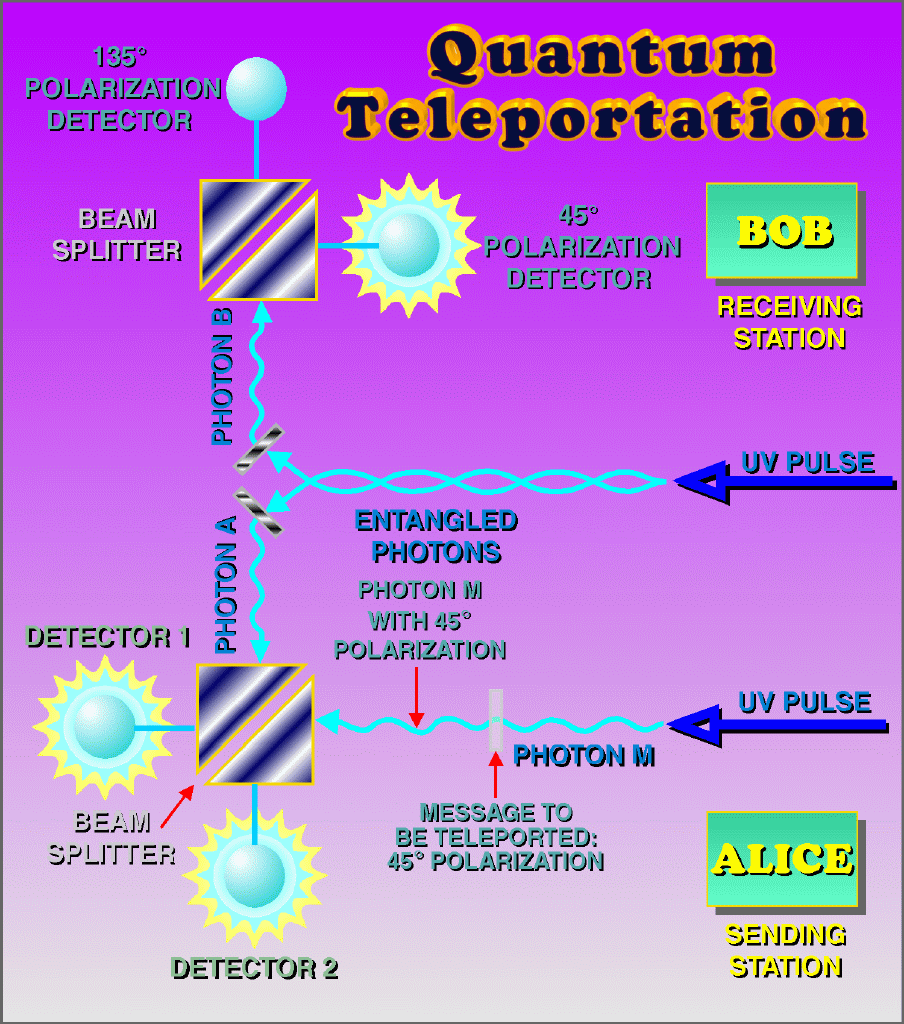
6.) Quantum mechanics enables teleportation. Quantum teleportation is indeed a real phenomenon, but it does not imply the physical teleportation of objects. By holding one of two entangled particles nearby and sending the other to a different location, the information regarding an unknown quantum state can be transmitted. However, this process is limited to single particles and pertains only to information about uncertain quantum states—not physical matter. The concept of teleporting a human being is thus impossible.
7.) All aspects of a quantum universe are uncertain. While certain elements are uncertain, many aspects are well-defined in a quantum universe. For example, regarding an electron, you cannot precisely know both its position and momentum or angular momentum in multiple directions simultaneously. Yet, some properties, such as rest mass and electric charge, can be precisely determined. Uncertainty in quantum physics typically arises between pairs of physical quantities related to one another, known as conjugate variables, which explains the uncertainty between energy and time, voltage and free charge, or angular momentum and position.

8.) Identical particles share the same mass. If you compare two identical particles—like protons or electrons—on a precise scale, they would always register the same mass. This holds true only for stable particles with infinite lifetimes. For unstable particles, like top quarks or Higgs bosons, measurements would yield varying results due to the inherent uncertainty between energy and time, leading to an uncertainty in mass.
9.) Einstein rejected quantum mechanics. Einstein's famous remark, “God does not play dice with the universe,” reflects his skepticism towards the inherent randomness in quantum mechanics. However, this skepticism pertains to interpretations rather than a dismissal of quantum mechanics itself. Einstein believed there might be more to the universe than what we can currently observe, suggesting that what appears random might reveal a deeper, non-random truth. While this viewpoint has not produced practical results, the examination of quantum fundamentals remains an active research area.
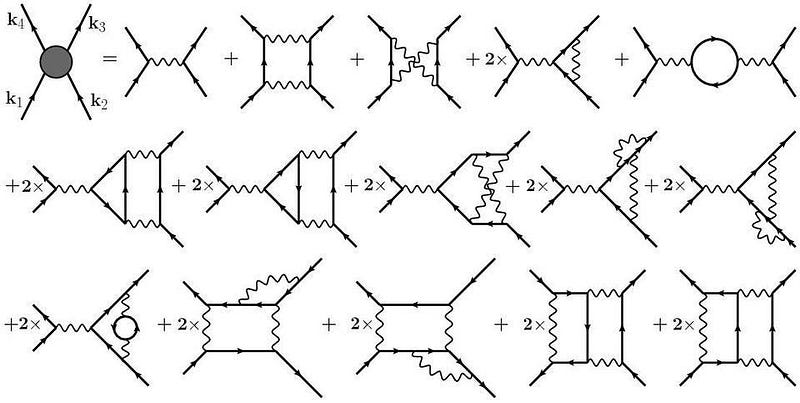
10.) Quantum field theory completely describes our universe through particle exchanges. In graduate school, physicists learn that the most common method for calculating interactions between quantum particles involves visualizing exchanges of particles. However, extrapolating this to encompass all possible interactions leads to nonsensical results. This technique is merely an approximation and breaks down after a certain number of terms. While the notion of virtual particle exchanges is compelling, it is fundamentally incomplete and unlikely to represent the final understanding.
Starts With A Bang is now featured on Forbes and republished on Medium with a 7-day delay. Ethan has authored two books, Beyond The Galaxy and Treknology: The Science of Star Trek from Tricorders to Warp Drive.