Understanding Statistical Mechanics: A Journey Through Molecules
Written on
Chapter 1: The Essence of Fluids and Their Properties
Fluids can be notoriously challenging to define. We often discuss temperature, humidity, and wind, but have you considered the underlying nature of these measurements? Surrounding us are countless molecules, and the sensations we experience in our environment arise from their collective behavior. For a long time, physicists grappled with how to articulate these properties in tangible terms. Although foundational concepts began to emerge in the 1700s, the formal development of statistical mechanics took off in the mid-1800s, providing a framework for explaining thermodynamic phenomena. In this article, we will explore the evolution of statistical mechanics and the fundamental ideas utilized by physicists to analyze vast collections of molecules.

Chapter 1.1: Historical Foundations
The roots of statistical mechanics can be traced back to Daniel Bernoulli, a prominent figure from the influential Bernoulli family, known for their contributions to mathematics and physics in the 17th and 18th centuries. Bernoulli introduced a gas theory referred to as the "billiard ball" model, which depicted gas as a multitude of tiny molecules colliding with one another, akin to billiard balls interacting on a table—though this occurs in three-dimensional space.
Building upon Bernoulli's groundwork, physicists such as Rudolf Clausius and James Clerk Maxwell made significant advancements in gas theory during the mid-1800s. This era was marked by a keen interest in thermodynamics and steam engine development. However, it was Ludwig Boltzmann who firmly established statistical mechanics, demonstrating its core principles and utilizing it to validate the second law of thermodynamics.
Chapter 1.2: Defining Statistical Mechanics
Statistical mechanics is frequently mistaken for thermodynamics. While both fields share similarities, they are distinct. Thermodynamics is an older discipline focused on the relationships between heat, work, temperature, and energy over time. In contrast, statistical mechanics emerged as a collection of techniques to elucidate thermodynamic concepts, such as Boltzmann's application of statistical mechanics to substantiate the second law of thermodynamics. It employs statistical methods to summarize the dynamics of numerous particles over time, which is essential for comprehending the vast number of molecules in a gas.
To illustrate a basic application of statistical mechanics, consider temperature. While we intuitively grasp temperature by touch, its scientific definition relates to the average squared velocity of molecules within a gas. The equation for this relationship may appear complex:
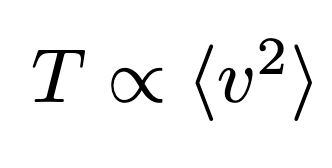
In this equation, the infinity symbol indicates proportionality, meaning changes on one side directly affect the other. The angular brackets signify averaging across the entire gas—squaring each particle's velocity and averaging those values. Although measuring each individual particle in a gas is impractical, this approach provides a useful conceptual framework.
Chapter 2: The Power of Statistical Mechanics
Statistical mechanics encompasses numerous concepts like the one discussed above. By starting with basic interactions among molecules and scaling them to represent extensive systems, we can derive significant insights that clarify various thermodynamic processes. One particularly intriguing aspect is the Maxwell-Boltzmann Distribution, which describes the distribution of molecular speeds in an ideal gas at a specific temperature. It's fascinating how we can encapsulate such a complex phenomenon with a relatively straightforward principle!
If you're interested in delving deeper into the mathematical aspects of statistical mechanics, I recommend checking out this free online textbook. It's both accessible and precise, assuming a foundational understanding of physics.
The first video, "ALEKS: Interconverting Number of Atoms and Mass of a Compound," provides a comprehensive overview of how to relate atomic quantities to compound mass through practical examples.
The second video, "How to Convert Moles to Number of Molecules," illustrates the conversion process between moles and molecules, offering clear explanations and visual aids.
I hope this exploration has been enlightening! If you appreciate my work, consider becoming a Medium member using this link to support me. Additionally, you can follow me or my publication, Science Pathways, for more engaging stories about mathematics and science, which I publish weekly.