The Fascinating Science of Evaporation and Its Everyday Impact
Written on
Chapter 1: Understanding Evaporation
Evaporation is often overlooked, yet it is a fundamental process that significantly influences our daily experiences. For example, have you ever pondered why mist appears on chilly winter mornings? This phenomenon occurs due to falling temperatures, which lead to the condensation of water vapor. Evaporation is not just about aesthetics; it is a vital mechanism in our lives.
Consider how we never need to pay for our wet clothes to dry—this is the magic of evaporation at work. On hot days, the evaporation of sweat helps keep us cool, while cooling towers utilize this process to manage the temperature of water used in electricity generation, ensuring we have power in our homes.
Grasping the significance of evaporation enhances our appreciation for its role in nature. You don't need advanced degrees to understand it; curiosity and mindfulness are all that’s required. Let’s delve into the atomic world, where everything is dynamic, bound by intermolecular forces that shape physical matter. Unlike solids, where these forces are strong, evaporation involves liquids and gases.
Picture a liquid filled with molecules dancing and shifting. Traditional explanations suggest that more energetic molecules escape into the gas phase, but this view is quite narrow. Instead, we should consider evaporation as a process of diffusion at the surface, which we will explore further.
Section 1.1: The Role of Diffusion in Evaporation
To comprehend evaporation better, we first need to understand diffusion, which describes how substances move from areas of high concentration to low concentration. When you spray perfume in a room, it gradually spreads to fill the space. Heat behaves similarly, transferring from hotter objects to cooler ones. Evaporation operates under the same principle, depending on the relative concentrations of liquid and vapor phases. This transfer continues until both phases achieve a state of equilibrium.
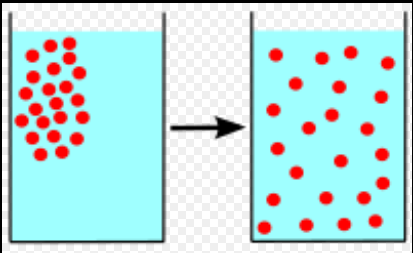
Section 1.2: The Mechanics of Evaporation
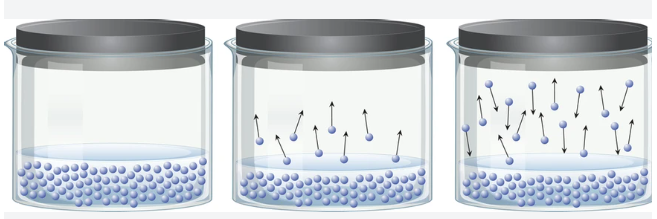
Let's visualize a hypothetical piston-cylinder system containing water at a pressure of 1 atm, achieved by placing weights on the piston. If we heat this system, the kinetic theory of gases tells us that the increased temperature causes the molecules to move more vigorously.
As the temperature rises, some molecules gain enough energy to break free from the water's surface and enter the vapor phase. Although we may not see anything above the water, measuring the pressure reveals the presence of vapor pressure resulting from evaporation.
When we reach 100°C, an intriguing phenomenon occurs: the internal vapor pressure matches the external pressure. Continuing to heat the system causes the piston to rise, even without adding more weights. This is because the vapor pressure equaling the external pressure signifies that both liquid and gas phases coexist, which is the boiling point.
The concept of phase equilibrium can be understood as the point at which the number of molecules escaping the liquid equals those condensing back into it. Although boiling and evaporation share similarities, they differ significantly; evaporation can occur at various temperatures, while boiling requires specific conditions.
Chapter 2: Environmental Influences on Evaporation
In real-world scenarios, evaporation is influenced by the relative humidity and temperature of the surrounding air. Think of the air as a container holding water vapor, contributing to a pressure known as vapor pressure.
Suppose the air temperature is 25°C with low relative humidity. The water in a beaker will continue to evaporate until it cools down, and the vapor pressure stabilizes at saturation pressure. At this point, evaporation halts, and boiling can commence if additional energy is provided.
Evaporation - Elementary Science - YouTube
This educational video provides an overview of evaporation, exploring its significance and the science behind it in a simple, engaging manner.
How Salt is Made | Evaporation Process & Facts - YouTube
This informative video explains the evaporation process involved in salt production, highlighting how evaporation plays a role in various industrial applications.
Conclusion
Evaporation, while appearing simple, is essential in our lives, from drying clothes to regulating temperature in industrial settings. By exploring the science behind this process, we gain a richer understanding of its importance in maintaining balance and efficiency across various systems. The next time you enjoy a refreshing breeze after sweating or notice your laundry drying, remember the incredible process of evaporation at work. Embrace your curiosity and continue to explore the natural wonders that often go unnoticed. By understanding these fundamental concepts, we deepen our knowledge and foster a greater appreciation for the intricate mechanisms that enhance our comfort and sustainability.