The Urgent Quest for Coronavirus Treatments and Vaccines
Written on
Chapter 1: Understanding the Current Situation
How will the coronavirus pandemic conclude? The response remains in flux. Although we can mitigate the virus's spread through stringent public health measures, it is likely to resurge as soon as those measures are relaxed. Until we have more effective treatments and a vaccine, life will remain challenging.
So, what’s the progress on this front? The following two infographics illustrate the current status of development as of March 20, 2020, based on reports from STAT.
Section 1.1: Current Treatments
The pursuit of treatments is crucial, as they may arrive sooner than vaccines. While these interventions won’t eradicate the virus, they could potentially save lives and ease the strain on healthcare systems.
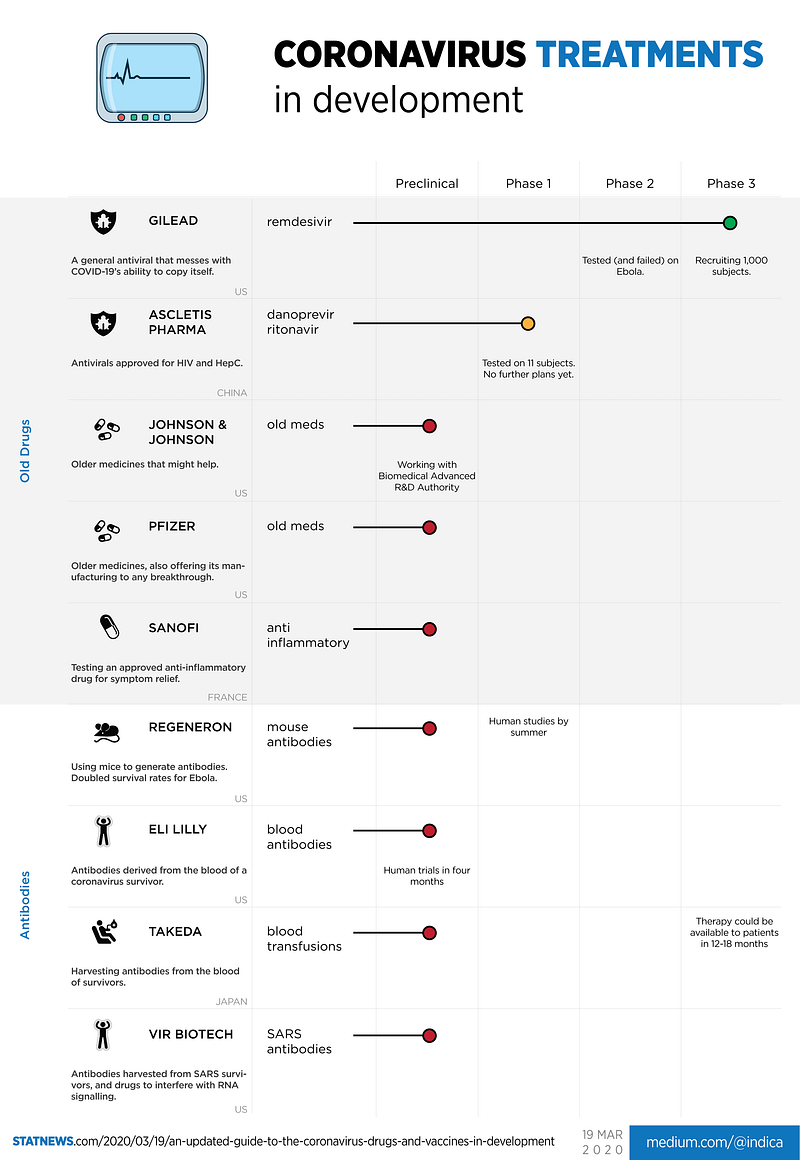
Treatment encompasses any approach that can save lives and alleviate the burden on healthcare. The prevailing research is focused on two main strategies: repurposing existing drugs and developing antibody therapies from recovered patients.
#### Subsection 1.1.1: Repurposing Existing Drugs
Pharmaceutical companies are exploring their existing inventories for potential treatments. The benefit of this approach is that these medications are already known to be safe, but they may not effectively combat the coronavirus.
Among these, Gilead's remdesivir is leading the way, currently in Phase 3 trials. This drug functions like a copier jam, theoretically hindering the virus's ability to replicate itself. However, its previous limited success against Ebola raises questions about its efficacy for COVID-19. Other companies, including Johnson & Johnson, Pfizer, and French firm Sanofi, are also investigating available options. Notably, Pfizer has offered to assist in manufacturing any successful breakthrough.
It is worth mentioning that I have excluded chloroquine and hydroxychloroquine from this discussion, as STAT did not include them. However, they have been referenced in separate articles, noting that the WHO is examining these medications.
#### Subsection 1.1.2: Antibody Development
Our bodies naturally produce antibodies to combat the coronavirus, facilitating recovery. Numerous treatments aim to expedite this process in various ways.
- Mice: Regeneron has developed proprietary mice that generate antibodies. Their treatments have shown promise in increasing survival rates for Ebola.
- Blood: Companies like Eli Lilly and Takeda are extracting antibodies from the blood of recovered COVID-19 patients, while Vir Biotechnology is utilizing blood from SARS survivors.
These treatments are primarily for patients already infected and may not be effective in advanced stages. Ultimately, the only definitive solution to this pandemic is a vaccine.
Section 1.2: The Path to Vaccination
To restore normalcy, a vaccine is essential. Life cannot resume as usual without it, and we must fundamentally rethink what "business as usual" entails.
The goal of these vaccines is to prompt our bodies to produce antibodies or provide the necessary information to fend off the virus.
#### Chapter 2: Vaccine Development Strategies
One promising method involves messenger RNA (mRNA), which conveys genetic instructions from DNA for protein synthesis. Scientists from Moderna, BioNTech, and CureVac are actively engaged in this research. In a controversial move, former President Trump reportedly attempted to secure CureVac's technology for exclusive U.S. use, a decision that overlooks the global nature of the pandemic.
Moderna is currently the most advanced, having entered Phase 1 trials with healthy volunteers this month. Typically, these phases take years, but if a viable treatment emerges, it could be expedited.
#### Subsection 2.1: Alternative Vaccine Approaches
Other research teams are employing deactivated or diminished COVID-19 components within harmless viruses to stimulate an immune response without causing the disease. Companies such as Cansino (China), Johnson & Johnson (U.S.), and Sanofi (France) are exploring this avenue. Cansino has recently received approval from Chinese authorities to commence human trials.
- RNA Vaccines: Arcturus (U.S.) is developing a synthetic vaccine utilizing RNA in a liquid nanoparticle form.
- DNA Vaccines: A collaboration between U.S. and Chinese entities, Innovio, is creating a DNA vaccine, with clinical trials set to begin in April and a commitment to produce 1 million doses this year.
- Protein-Based Vaccines: Collaborations between GlaxoSmithKline and Clover (China) aim to directly synthesize the proteins required for an immune response.
Conclusion: The Timeline Ahead
While we may see treatments emerge in a matter of months, a functional vaccine will likely take at least a year or more. The global economy is heavily reliant on these developments, which will attract funding and focus, but the scientific process cannot be rushed, especially given the novel circumstances we face.
If you’re wondering when these solutions will arrive, the answer is uncertain. I keep up with the latest reports from STAT and remain hopeful. We also lack clarity on production, distribution, and pricing.
Regardless of the cost, a successful vaccine is the only viable path forward. While some have suggested allowing widespread infection, this could lead to millions of deaths, and we cannot predict the duration of immunity that would follow. Abandoning our fight against the virus is not an option.
Currently, the most viable strategy is strict suppression, but this approach also hampers the economy and plunges countless individuals into poverty, which can lead to additional fatalities. We need to overcome this crisis, and for that, we urgently require effective treatments and vaccines.